Enoxaparin Sodium
CAS No.: 679809-58-6 (Low molecular weight heparin)
Specification
| Enoxaparin Sodium | ||||
| Source | Intestinal mucosa of pigs | |||
| Quality standard |
USP |
EP |
||
|
characters |
Appearance |
/ |
white or almost white; hygroscopic powder | |
| solubility |
/ |
freely soluble in water | ||
| identification | The spectra exhibit maxima at 231±2nm |
The 13C NMR spectrum obtained is similar to that obtained with the appropriate specific Enoxaparin sodium CRS |
||
| The spectra are similar |
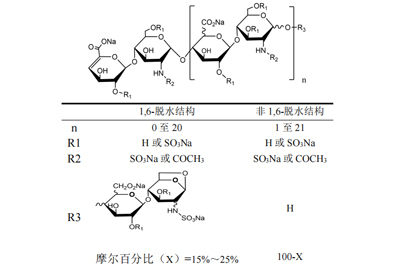
15 -25 % bearing the 1,6-anhydro structure at the reducing end of their chain15%- 25 % of components bearing the 1,6-anhydro structure at the reducing end of their chain |
|||
|
Anti-factor Xa activity/ anti-factor IIa : 3.3-5.3 |
Anti-factor Xa activity/ anti-factor IIa : 3.3-5.3 |
|||
|
Mw : 3800 -5000Da, M≤2000 : 12.0%-20.0%, M≥8000:NMT 18.0%, M2000-8000:68.0%-82.0% |
Mw : 3800 -5000Da, M≤2000 :12.0%-20.0%, M2000-8000:68.0%-82.0% |
|||
|
Meets the requirements for Sodium chemical identification tests |
It complies with the test for sodium | |||
| Appearance of solution | / |
clear;NMT: intensity 6 |
||
| Specific Absorbance | 14.0-20.0 (on the dried basis) |
14.0-20.0 (on the dried basis),determined at 231nm |
||
| pH |
6.2-7.7 |
6.2-7.7 |
||
| Benzyl alcohol |
≤ 0.1% |
≤ 0.1% |
||
| Molar ratio of Sulfate carboxylate | ≥ 1.8 | ≥ 1.8 | ||
| Nitrogen |
1.8 %-2.5 % (on the dried basis) |
1.5 %-2.5 % (on the dried basis) |
||
| Sodium | 11.3% -13.5% (on the dried basis) |
11.3% -13.5% (on the dried basis) |
||
| Loss on drying |
≤ 10.0% |
≤ 10.0% |
||
| Bacterial endotoxins |
≤0.01EU/U |
≤0.01EU/IU |
||
| Assay | Anti- factor Xa activity |
90-125 IU/mg (on the dried basis) |
90-125 IU/mg (on the dried basis) |
|
| Anti- factor IIa activity |
20.0-35.0 IU/mg (on the dried basis) |
20.0-35.0 IU/mg (on the dried basis) |
||
Indication
1. Prevention of venous thromboembolic diseases (prevention of venous thrombosis), especially in relation to orthopaedic or general surgery
2. Treatment of existing deep vein embolism, with or without pulmonary embolism, with mild clinical symptoms, excluding pulmonary embolism requiring surgery or thrombolytic therapy.
3. Treatment of unstable angina pectoris and non-Q wave myocardial infarction, combined with aspirin.
4. Used in cardiopulmonary bypass for hemodialysis to prevent thrombosis.
CAS No.: 9041-08-1 (Low molecular weight heparin)
CAS No.: 37270-89-6 (Low molecular weight heparin)